
SARS-CoV-2
Detection Kit
Fast and simple COVID-19 diagnosis through minimized procedure

Fast and simple COVID-19 diagnosis through minimized procedure


| Item | Quantity / Volume | Description | Storage |
|---|---|---|---|
| Premix | 5 tubes(250 μL each) | Includes RTase for one-step RT-PCR | Store at below -20℃ |
| DNA/Rnase Free Water | 5 tubes(50 μL each) | For running controls | |
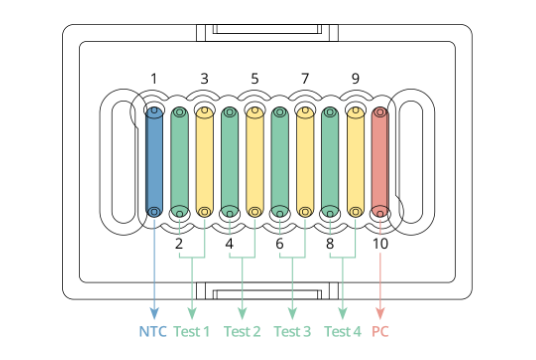
| Test Chip | 25 EA | With labeled primers and probes | |
| Sealing Tapes | 25 EA | For sealing the wholes of test chip after loading reaction mixtures | |
| 5-strip Tubes | 25 EA | 0.2mL (For mixing premix with template) |
| Cat. No. | Product | Pack Size |
|---|---|---|
| 9799151403 | SMARTCHEK® SARS-CoV-2 Detection Kit | 100 tests / PK (25 test chips / PK) |
| 1399100200 | GENECHECKER® UF-300 Real-time PCR System | 1 SET |
| 1499100100 | GENECHECKER® UF-340 Four-in-One Real-time PCR System | 1 SET |
Please select a product category.
Genesystem’s website stores and retrieves visitor information in the form of cookies to enhance user experience.
Genesystem respects visitors' rights to data privacy. Visitors can review detailed information in the cookie policy and can choose not to allow specific types of cookies through the cookie settings below.